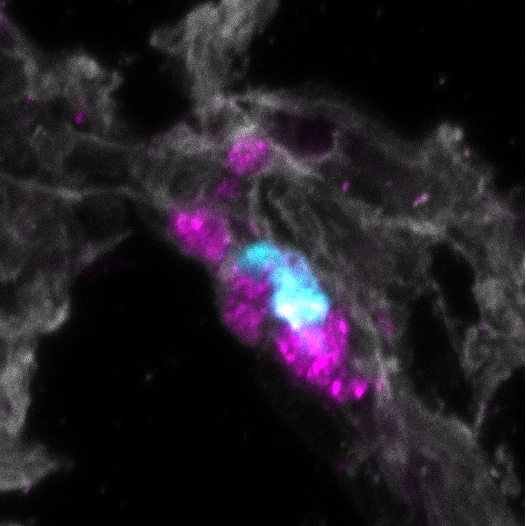
Singular tumor cell (blue) covered in platelets (magenta)
Despite being a reservoir for pro-angiogenic and metastatic cytokines, platelets also contain negative regulators of tumor progression. An ongoing project in the Battinelli lab explores the contributions of platelet angiopoietin-1 in protecting against tumor cell metastasis. Using pre-clinical models, the project explores how platelet Angpt1 limits tumor cell interactions with lung endothelial cells, reducing tumor cell extravasation and survival. This project provides a rare example where platelets contain and release a negative regulator of tumor metastasis and highlight the complexity underpinning the communication between platelets and cancer.
Although immune checkpoint inhibitors (ICIs) offer promise in several malignancies; immune refractoriness remains an area of unmet need in cancer. A deeper understanding of immune regulation within the complex tumor microenvironment (TME) and how to promote anti-tumor immunity would advance our ability to achieve a durable anti-tumor immune response. One commonly overlooked component of the TME that has consistently been associated with promoting tumor growth and metastasis is the platelet, a cell best known for its role in hemostasis, but increasingly recognized for its contributions to a variety of immune responses.3 Our laboratory has been instrumental in establishing platelet and tumor cell interaction as a chief regulator of metastatic spread of breast cancer. My laboratory has recently identified a new mechanism used by platelets to aid malignancy by helping tumor cells to avoid immune surveillance through regulation of the immune checkpoint protein, programmed death-ligand 1 (PD-L1) on cancer cells. Our innovation builds upon our hypothesis that platelets promote cancer by upregulating PD-L1 in tumor cells enabling them to evade immune surveillance. Our discoveries strongly support a new paradigm of modulating platelets to render the tumor cells sensitive to ICI treatment with improved outcomes.
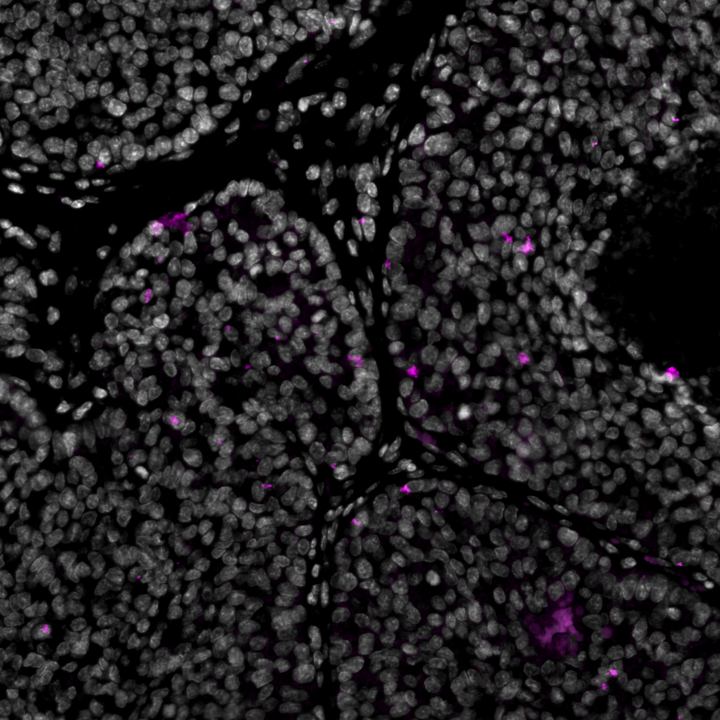
Infiltrating CD4+ lymphocytes within the mammary tumor of a mouse
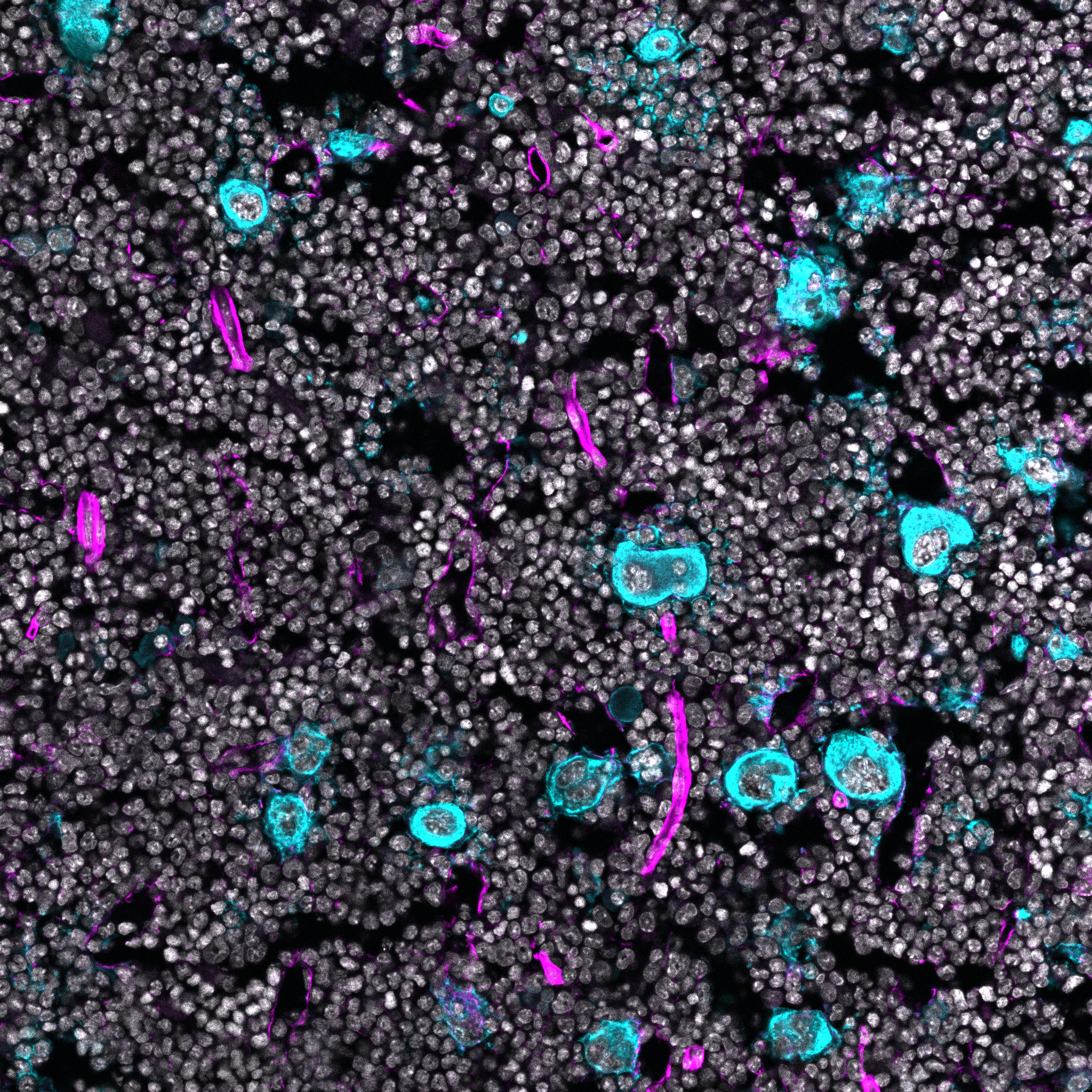
Confocal image of bone marrow in breast cancer, depicting megakaryocytes (cyan) and blood vessels (magenta). Image taken by Dr. Isabelle Becker.
Platelets are produced by megakaryocytes that predominantly reside within the bone marrow. We aim to determine whether malignancy influences megakaryocyte maturation and platelet production within the bone marrow milieu. We believe that megakaryocytes in the setting of cancer are reprogrammed and undergo abnormal thrombopoiesis, producing platelets that look fundamentally different to those made in healthy individuals.
We aim to understand the molecular crosstalk that occurs between platelets and tumor cells to determine 1) how platelets become activated in response to tumor cells and 2) how interaction with platelets regulates the metastatic phenotype of tumor cells. Specifically, we are interested the immune regulatory function of platelets to tumor cells. We found that platelet upregulate tumor cell PD-L1 level in EGFR dependent way. Given the recent clinical trials establishing that anti-platelet agents such as aspirin can improve survival and decrease metastatic spread in some cancer patients, we also aim to understand how anti-platelet therapies disrupt the effects of platelet to tumor cells.
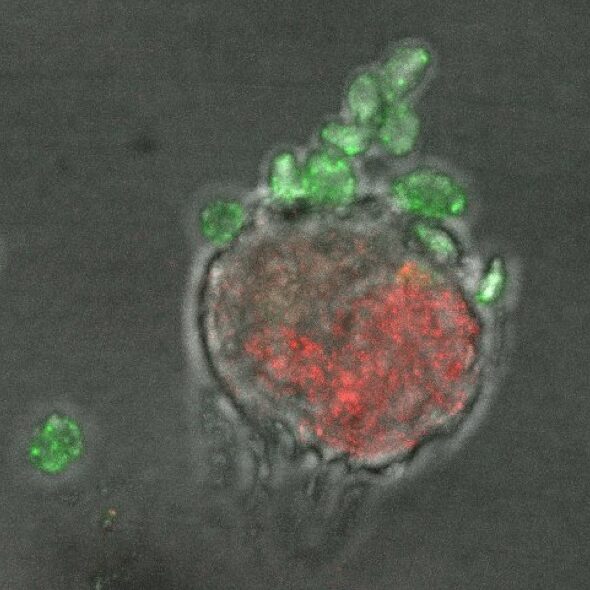
Confocal image of a tumor cell (red) interacting with platelets (green).
Battinelli, E. M., Thon, J. N., Okazaki, R., Peters, C. G., Vijey, P., Wilkie, A. R., Noetzli, L. J., Flaumenhaft, R., & Italiano, J. E. (2019). Megakaryocytes package contents into separate α-granules that are differentially distributed in platelets. Blood advances, 3(20), 3092–3098. https://doi.org/10.1182/bloodadvances.2018020834.
Roweth, H. G., Malloy, M., Forward, J. A., Ceglowski, J., Flaumenhaft, R. C., Italiano Jr, J. E., & Battinelli, E. M. (2019). The Effects of Antiplatelet Agents on Endocytosis. Blood, 134, 1058. https://doi.org/10.1182/blood-2019-131912